Tooth whitening
Many years dentistry is trying to develop and propose means to the patients for teeth whitening. The reason is the desire of more people to have teeth with a lighter color. White teeth are perceived aesthetically better than others, they are a sign of good health and high social status. The community talks about so-called Hollywood smile - clearly arranged teeth with a healthy crown with no inflammation and, last but not least, of a lighter color. Very often when we have been called upon to choose the color of teeth with restorations we had to convince patients not to put the brightest possible color - it shows how relevant the presence of recently acquired white teeth for the confidence of people is. Before proceeding to the teeth whitening it is important to clarify the reason for the coloring, but also to diagnose the state of dental pulp. Discoloration may occur in vital teeth and non-vital ones, respectively, in such cases we talk of exo - or endogenous staining. The exterior color is influences by some substances that stain or damage the surface of the teeth enamel.
Attempts at bleaching vital teeth are made from the late XIX century. Initially, the dentists were trying to find the most effective bleaching agent. Attempts have been made with oxalic acid, then - with chlorine and calcium hypochlorite. From 1884 onwards most widely used is perhidrol (concentrated hydrogene peroxide). Subsequently introduced are some systems based on karbamid-peroxide but nowadays still most whitening systems are with an active ingredient perhidrol. In 1895 it was first attempted by electrophoresis to accelerate the process of bleaching; in 1911 it was made with ultraviolet rays, but subsequently the method was abandoned due to the strong adverse effects of this radiation. From 1918 (exactly one year after the Great Socialist Revolution Oktomriyska ) as the gold standard in the world dentists begin to apply bleaching perhidrol in combination with heat or light rays to accelerate the process.
www.ralev-dental.com www.bg-dentist.com
In general, modern dental whitening systems are of two types: for domestic use and for use in-office (in a dental cabinet). Household bleach systems are applied in specially made plastic trays (much like boxing bites) that the patient has at home during the day or night. Professional whitening systems are applied once in office - their effect is obtained more quickly, within one to two hours. The team in our dental practice more manifest preference for in-office bleaching systems application. They have a very significant advantage - before aplication of the whitening system professional dental hygiene is implemented - tarter, plaque and stains of coffee and cigarettes are proffecionally removed. This is not so easily achievable at home - it is always possible to remain uncleaned portion of the teeth, which worsens the effects of applications of whitening gel. Moreover, professional whitening systems allow to achieve a rapid effect - it is not irrelevant today, when consciousness is involved with many things simultaneously. Sometimes patients simply forget to put buses with whitening gel, which worsens the effect. Moreover, most people prefer whitening their teeth to be achieved the fastest possible way - in upcoming shows, important personal occasions, etc. For example, for a period of nearly ten years since bleaching teeth, we observe an interesting subject - increased number of patients whitening around prom and graduation nights (15.05 - 30.05. Each year) and around the New Year.
How exactly are teeth whitened? It is possible to do due to the permeability of hard dental tissues to hydrogen peroxide. Generally, hydrogen peroxide is a very reactive compound and when activated quickly begans to crumble to water and oxygen radicals. The later quickly penetrate between the enamel prisms and dentinal tubules especially. There are soaked pigments there, whose molecules are torn by oxygen radicals into smaller fragments and are drawn out by diffusion. This is the mechanism of action of all whitening systems that are currently produced in the world - clinical, home, for devitalized teeth whitening, etc. The process of decomposition of hydrogen peroxide is strongly influenced by temperature - for example, at elevated temperature by 10 degrees the rate of decay is increased twice. Furthermore, the higher temperature accelerates the process of diffusion of oxygen radicals because of the accelerated Brownian motion of particles. Here is the main disadvantage of bleaching systems - the higher temperature is not very positively perceived by the patient, and also leads to worsening of the composure and qualities of the product. It is better to work only with fresh solutions in shelf life and once bought bleaching system is good to keep in the refrigerator.
In the older literature we have found reports of good
effect of diethyl ether, when mixed with bleaching gel - the authors claim it
increased permeability of the enamel to bleach. Lately we have not met
scientific reports of such methods - possibly at higher concentrations of the
ether anesthetic effect of ether occur with any adverse effects on the patient
and dentist. Hydrochloric and hydrofluoric acid can also be used to increase
bleach power, though most authors say it is too aggressive - it should be used
most often in severe fluorosis.
Address of Practice - Dental Practice Dental Ralev - town
Plovdiv Keep your appointment for teeth whitening by phone +359 32
642056 Address of Practice - Dental Center Omegadentagrup - town
Vidin Whitening system whitening system teeth whitening devitalized
teeth How to whiten teeth? Caries Endodontics Dentistry Mechanism of action of bleaching systems?
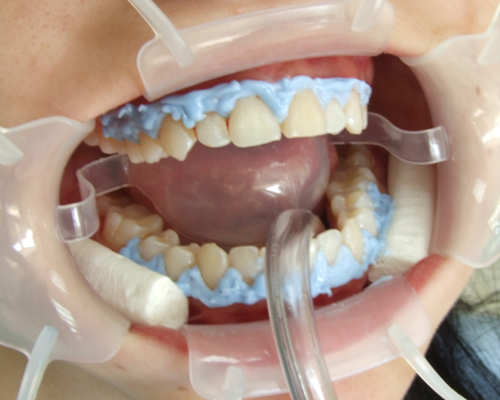
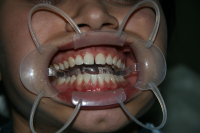
Available several
photos here present the case of teeth whitening using the system Pola office
product of the Australian company SDI . This system is with one of the highest
concentrations available on the market - 36% hydrogen peroxide. The active
ingredient in all whitening systems is inorganic substance hydrogen peroxide. In
some whitening products there is also karbamidperoxide - a substance which,
under irradiation with blue light activates and enhances the bleaching action of
the basic agent. It is important to isolate the gingiva from the effects of
bleaching system. This is done using a gingival barrier of light-cured plastic -
it is transferred onto the gum and is irradiated with light, and hardenned. If
not protected this can lead to chemical burns of the gingival tissue - something
extremely unpleasant as a sensation. With careful use of the whitening systems
and better insulation of the gums such complications are rare - if still a small
amount of bleaching gel gets on the gum, it is cleaned quickly and the site
remains whitish color which passes in a few hours.* www.bg-tourinfo.com
www.investor-bg.com
www.omegadentagroup.com Free on-line dental consultations Want to have white teeth? Save your time for consultation and
possible bleaching phone +359 32 642056
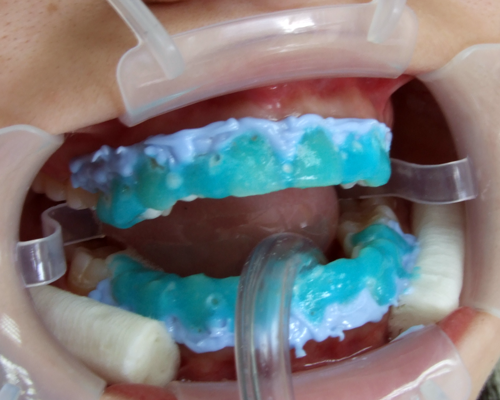
Pictured left is seen the bleaching gel after aplication.
Manufacturer intentionally had it colored in cyan - so that the dentist can see
where the system is aplied and protects the gums from damage. This is not
irrelevant, since the bleaching is a very demanding operation, in which the eyes
of the dentist tire and often colorless bleaching gels are not visible if it
falls on the gum. What is the principle of action of the bleaching of teeth?
To explain this an accessible level, it is necessary to explain why the color of
natural teeth changes. This is an ongoing process that occurs throughout the
life of man, due to infiltration of pigments between enamelling prisms. Coloring
agents are everywhere in the composition of foods and beverages - particularly
aggressive in this respect are coffee, black tea, red wine and chocolates.
Besides these staining of teeth can cause many other products: juices
(blueberry, black currant, etc.), confectionery colors for cakes, some herbal
extracts (sumac), jams, purees and what not. The food industry has recently
increasingly relies on organic chemistry to improve the quality of food.
For teeth whitening hydrogen peroxide penetrates between enamelling prisms and dissolves colors available there. The darker the teeth are
in a patient, the more aggressive must be action upon them - the reason for the
darker color is the greater amount of coloring agents. In such cases, it is
recomended leaving the gel on the teeth for a bit longer, so he was able to get
the colors more effectively. This is also an indication for the application of
bleaching system with a high concentration of hydrogen peroxide. In the bleaching of teeth there are some
contraindications. In patients with prosthetic restorations on anterior teeth
results are unpredictable - dental tissues are bleached and crowns and fillings
are not and therefore not able to obtain color difference. If necessary,
replacement of restorations is best done after first bleached teeth, waiting for
a short period of time (ideally about two weeks) and only then draw the new
crowns and fillings. The reason is the residual effect of bleaching systems -
even after the manipulation interprismatic spaces remain open, colors are
separated out and this whitens your teeth more. This process takes place mostly
during the first 72 hours, but according to most manufacturers is best to wait
about two weeks to achieve the final result. Cost of teeth whitening in dental offices Dr. Ralev - town
Plovdiv - 200 BGN to once in office bleaching Cost of teeth whitening in Dental Centre Omegadentagrup
- town Vidin - 80 BGN for a single bleaching in the office Warm vertical condensation of the root canal www.endodontia.bg
www.dentalimplants.bg
www.omegadentagroup.net
The photographs below shows the clinical case of whitening
teeth whitening system with American *ContrastAM *the manufacturer. Spectrum
Dental. This was the first product that was introduced in the Bulgarian market
from the newly registered company Ralev Dental Corp.. The first patient to
undergo the procedure in question was a student of dentistry, which came to
study in our clinical practice at the time. Everything was extremely exciting
for our team: we had bought something from halfway around the world that we
intended to offer to all our colleagues across the country; we contacted the
manufacturer during the Congress of theFDI in the emirate of Dubai in 2007.
Several months later completed the first direct import of dental materials and
immediately subjected the product to full clinical trials. The result obtained
was quite good, until then, we had only clinical experience with the whitening
system Pola office of SDI - Australia. Immediately appreciated the benefits of
the product of our new trade partner - a syringe whitening gel was constructed
so as to accurately mesure the ratio of two components; gingival barrier was
very homogeneous and solid, with excellent adhesion to pre-dried dental gingiva.
In the version of thePola Office, which we used until then, we had to pre-mix
powder and liquid. Their ratio had to be fixed by the eye of the dentist, as a
result, obtained either too thick or too thin consistency. If too thick the
whitening gel's effect of bleaching is not as good, too rare and the bleaching
gel action is excellent, but the gel ran out of the mouth of the patient and
began to cash in gum and oral mucosa. All this is avoided in the system
ContrastAM.
www.ralev.biz
www.ralev.name
www.markova.info
www.markov.ws
www.ralev.ws
www.simeonov.ws
www.buchkova.com Commercially we often hear about other bleaching products -
besides the conventional bleach systems there are whitening toothpastes, gargle
with Arcol water, etc. We have not seen a visible effect on the use of
toothpaste or mouthwash; anyway, these products will not damage the surface of
the tooth. It is danegrous to use some outdated methods of teeth whitening - rub
the enamel with lemon peel, use of different abrasives, etc. In such methods
there is further wear of the already tapered enamel colored teeth. Never resort
to self-treatment and always consult a dentist if you want whiter teeth - the
dentist can achieve a lighter color without damaging in any way dental tissues. Maxillo - facial surgery Sofia Dental Meeting Tooth Dentistry Tooth Decay
endodontics. Online shop for dental materials Investor Relations Dental
Implants Composite pins Keep your appointment for teeth whitening by phone 032 642056
we will try to maximize the light color of your teeth! For the first time in 1950 it was reported staining of
teeth in children as a result of taking tetracycline. As a result of the
widespread use of this antibiotic there is a rapidly increasing number of cases
with this staining, which is definitely becoming a problem as tetracycline
staining remains for life. In general, such coloring is obtained by taking tetracyclines in doses over one gram daily in pregnant women and children up to
age 8. There are more than 2000 tetracycline derivatives patented, some of which
still apply as an indispensable means - such as chronic bronchitis, cystic
fibrosis. Teeth are sensitive to tetracycline during their development, during
which the tetracycline molecule chelate with calcium and is involved in forming
the faulty coloured hydroxilapatite crystal. It stains mineralized tissues -
enamel and dentin. Coloration may be more or less pronounced, depending on dose,
type of preparation, patient age and duration of treatment. The color of teeth
can vary from yellow - orange to dark gray - bluish tint. Depending on the
severity of staining and prognosis differ four levels of shading: I
- yellow or light brown color evenly without streaks and spots II
- gray color, more intense, but also uniformly III
- dark gray or blue color, bands of weaker and more pronounced discoloration IV
- strong color, dark blue to black In the most general case tetracycline staining is difficult to remove by the
method of whitening teeth - enamel is not with a normal structure, prisms are
not properly located, there are globules, blades, other deposits, etc. In such a
construction of the enamel whitening gel can not penetrate deeply and bleaching
is not as effective for a scheme of action of bleaching systems click here ...
Durable and reliable result is obtained by making porcelain veneers . In the
first or second degree teeth may be in part bleached by harder application of
whitening systems.Fluorosis is also a problematic medical condition regarding the color of
teeth, produced if fluorine content in drinking water isover 1,5 ppm. At
concentrations above 4ppm serious damage to tooth enamel is observed that
manifests clinically with strong staining of the tooth crown. In different
periods in our history endemic outbreaks of fluorosis was observed in Burgas ,
Velingrad, Hissar and other cities - in establishing the risk of fluorosis
authorities are trying to take measures and sooner or later there is change of
the primary water source in these locations. In the Bulgarian state
administration and operation of municipal authorities, this is almost always
done late or very late. High concentrations of fluoride causes metabolic
disturbances in ameloblastite forming a defective matrix is followed by
irregular calcification and alternating areas of hypo-and hipermineralization.
There are two types of changes: internal colorimg and blemishes - only with
surface defects it is likely for bleaching procedure to be successful, while
internal staining results are discouraging - the teeth are not affected. The
literature describes three degrees of fluorosis:- I grade - brown pigmentation
of the smooth enamel surface, it is possible to bleach
- II level - flat gray or white
spots on the teeth surface. Such teeth are bleached with difficulties because it
cannot be obtained the same effect as with whitening stains and other surface.
- III degree - brown colored
blemishes. Darker pigmented defects are bleached quite hardwhich is why in many
cases it is appropriate bleaching to be combined with obturation with composite
materials.
There are also stains after
haemorrhage in the pulp. In severe trauma there are torn blood vessels inside
the pulp chamber which leads to internal bleeding. Blood enters the dentinal
tubules by capillary action; erythrocytes disintegrate and release hemoglobin.
Iron is released, which is associated with the available hydrogen sulfide and
ferrous sulphate is formed with a very nasty black color. Sometimes we can even
see the classic stages of absorption of the hematoma - originally the crown is
blue, then orange, yellow, light yellow and finally it takes the normal color.
In all cases, these teeth are bleached with a very good result by using the
endogenous methods of bleaching - for more details read here ... When necrosis
of the pulp for some reason occurs the results are the same - change of color of
the tooth and we have to do internal bleaching. All derivatives of necrosis lead
to brownish coloring. Described are the different biochemical mechanisms of
formation of pigments - for example, tryptophan (an amino acid that is a normal
component of protein molecules) is metabolized to melanin. The more time the
pigments reside in the pulp cavity, the more difficult it is to bleach the tooth
afterwards. In younger patients the results are better and faster due to better
permeability of hard dental tissues - dentinal tubules are wider. Absorption of
pigments from food or tobacco smoke increases the blackening of the teeth and
worsen the prognosis in terms of bleaching. In many cases, we are talking
about iatrogenic staining of the teeth - improperly performed medical
procedures, resulting in the re-formeing of pigments and their infusion into the
dentinal tubules. Vital extirpation or amputation for example, can lead to
bleeding, which staines teeth as per the described above mechanism. If left
residual pulp tissue in the pulp chamber "horns" this could lead to its
disintegration and subsequent gangrenous greyish-black staining of teeth. A
number of medications and filling material can lead to different color shaded
crown. For example, silver nitrate, which is sometimes used in children's
dentistry, causes blue-to-black staining, iodine staines the crown of the tooth in orange, yellow or brown, and metallic pins and
fillings - in dark grey. In the past
the filling paste Foredent of Spofa Dental was widely applied, which is
formalin-rezorcin based material and stained teeth in a pinkish color. Similar
results when using the SPAD root canal paste. Corrosion products with the use of
metals for direct restorations also cause iatrogenic coloring - amalgam turns
dental tissues grey to black and metal pins - in bluish-black. It is better to
build dental stumps using composite pins.
In all these cases the duration of action of the coloring matter
determines the depth of penetration, the degree of staining of teeth and
possibilities for its bleaching. In various cases prognosis varies greatly -
sometimes we manage to bleach severly stained teeth, in other cases less colored
teeth resist bleaching and the resurlts are insignificant. Description of
methods for devitalized teeth whitening is posted here ...In some rare common diseases
pathology can lead to staining of teeth by diffusion of various pigments in
dentin. For example, in hemolytic disease of the newborn (inRh- incompatible
pregnancy) decomposition of erythrocytes of the fetus leads, along with all
other problems, to the newly formed dental embryos to become stained. These
children have severe systemic and organ damage and discoloration of the teeth is
their last problem, but found in the literature are descriptions of similar
cases. Newborns with severe jaundice may later have teeth, stained blue-green or
brown. This is due to experience postnatal bilirubin or biliverdin in the hard
tooth tissues. Porphyria creates pigments that stain teeth reddish-brown color.
It is very difficult to whiten
teeth that are stained due to hereditary diseases - amelogenesis imperfecta
hereditaria ,dentinogenesis imperfecta hereditaria etc. In such diseases there
is impaired matrix and mineral component of enamel and dentin, and bleaching is
largely meaningless - either way, eventually the desease is leading to
construction of bridges and crowns or placing dental implants . Localized
staining of individual teeth may be due to defects in enamel and dentin,
resulting during the formation of teeth for example often occurs jyper - mineralized
white stain that with good oral hygiene has very good prognosis a few months
to a year away and it never reappears. These spots are very common in patients
with orthodontic appliances where cleaning is difficult in the cervical area.
Unfortunately, these colors are sometimes the cause of diagnostic errors and
unjustified use of whitening systems - in this case is necessary remineralizing
therapy rather than teeth whitening.Interesting physiological
changes occur with advancing age. The teeth become more yellowish due to
irreversible thinning of enamel, resulting in dentin starting to shine through.
In parallel, the tissues of the tooth seep multiple pigments - from cigarettes,
coffee, chocolate, red wine - things that are very nice, but amoral or fattening
... This makes the process of whitening teeth very difficult
and requires the use of more aggressive systems good choice is the
Italian system the Smile, in which the concentration of peroxide is 37.5%.